

|
| Home | Research | Publications | Recent news | Group | Teaching |
|
Electrolyte materials for solid oxide fuel cells researchPotential fitting for electrolyte materialsCerium dioxide, CeO2 or ceria, is an important material which has found applications in solid oxide fuel cells (SOFCs). Doping ceria with aliovalent cations, such as Gd, Sm, Y or La, leads to high ionic conductivity in the intermediate temperature range (500-800°C), thus raising the prospects of ceria-based electrolytes for application in SOFCs. The parameterization of an interionic potential for stoichiometric, CeO2 doped with a range of rare-earth dopants has been carried out. We use a dipole polarizable potential (DIPPIM: the dipole polarizable ion model) and optimize its parameters by fitting them to a series of density functional theory calculations. The resulting potentials were tested by calculating a series of fundamental properties for CeO2 and by comparing them to experimental values. The values for all the calculated properties (thermal and chemical expansion coefficients, lattice parameters, oxygen migration energies, local crystalline structure and elastic constants) are within 10-15% of the experiment, an accuracy comparable to that of ab initio calculations. This result suggests the use of this new potential for reliably predicting atomic scale properties of CeO2 and doped CeO2 in problems where ab initio calculations are not feasible due to their size limitations, such as surfaces and grain boundaries of electrolyte materials for SOFCs. 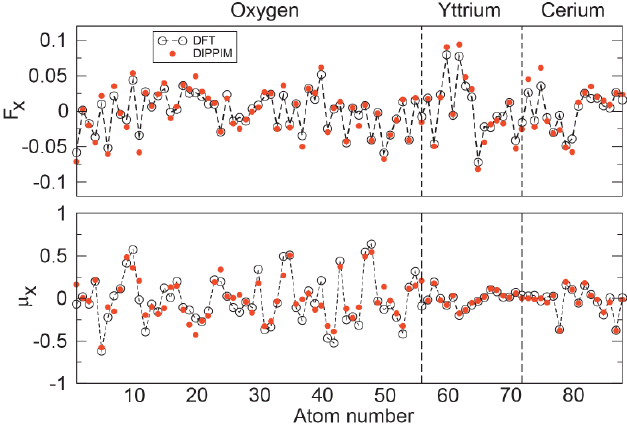
related references:
Vacancy ordering in Ce1-xYxO2-x/2The defect structure and ionic diffusion processes within the anion-deficient, fluorite structured system Ce1-xYxO2-x/2 have been investigated within the intermediate temperature range for SOFCs (500-800°C). Using a combination of neutron diffraction studies, impedance spectroscopy measurements, and molecular dynamics (MD) simulations, the defect structure and ionic conductivity of this material was studied. Particular attention has been paid to the short-range ion-ion correlations, with no strong evidence that the anion vacancies prefer, at high temperature, to reside in the vicinity of either cationic species. However, the vacancy-vacancy interactions play a more important role, with preferential ordering of vacancy pairs along the <111> directions, driven by their strong repulsion at closer distances, becoming dominant at high values of x. This effect explains the presence of a maximum in the ionic conductivity in the intermediate temperature range as a function of increasing x, which is a key factor in optimization of these materials for SOFC applications. This work was carried out with collaborators at the ISIS Facility in the United Kingdom and the Chalmers University of Technology in Sweden. 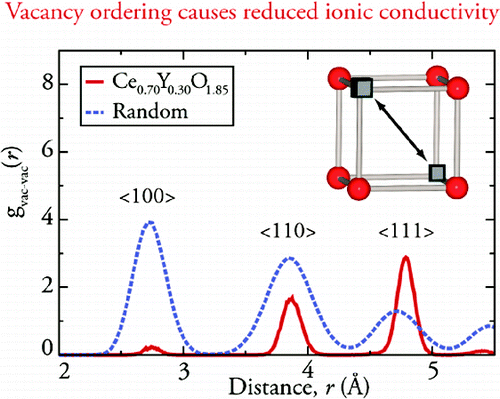
related references:
Strain effects in Ce1-xYxO2-x/2Strain induced in the growth of thin films can govern many properties of the materials. Molecular dynamics simulations on the effect of strain in Ce1-xYxO2-x/2 were performed under realistic operational temperatures and strain levels for SOFC applications. For bulk and thin film Ce1-xYxO2-x/2, we have shown that tensile strain leads to conductivity enhancements of up to 3.5x and 1.44x, respectively. The magnitude of these enhancements is in agreement with recent experimental and computational evidence. 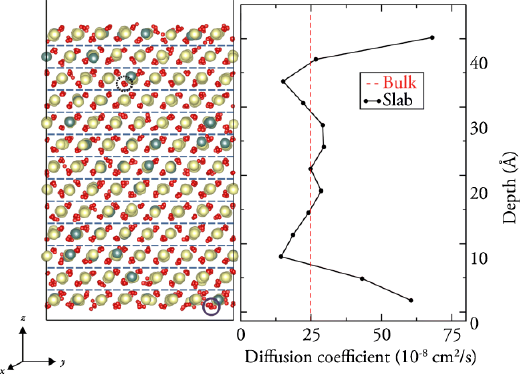
related references:
Effect of co-doping in CeO2Ceria co-doping has been suggested as a means to achieve ionic conductivities that are significantly higher than those in singly doped systems. Rekindled interest in this topic over the last decade has given rise to claims of much improved performance. Here we made use of computer simulations to investigate the bulk ionic conductivity of rare earth (RE) doped ceria, where RE = Sc, Gd, Sm, Nd and La. The results from the singly doped systems are compared to those from ceria co-doped with Nd/Sm and Sc/La. The pattern that emerges from the conductivity data is consistent with the dominance of local lattice strains from individual defects, rather than the synergistic co-doping effect reported recently, and as a result, no enhancement in the conductivity of co-doped samples is observed. 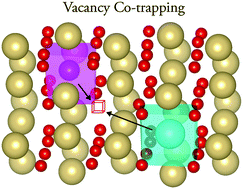
related references:
Email: watsong AT tcd.ie Last updated: May 25 2016 Back to Top |