

|
| Home | Research | Publications | Recent news | Group | Teaching |
|
Cathode materials for solid oxide fuel cells researchOxygen reduction reactionAt the cathode of a solid oxide fuel cell, oxygen is reduced, generating oxide ions, which are incorporated into the electrolyte. Perovskite-based materials have been used as SOFC cathodes due to good activity for the oxygen reduction reaction, high electrical conductivity and compatibility with electrolyte materials. Due to the poor oxygen ion conductivity of the cathode at intermediate temperature, the oxygen reduction reaction and incorporation of oxygen into the electrolyte is often limited to taking place at the interface between the cathode, electrolyte, and the air (the three-phase boundary). Mixed ionic and electronic conductors can be used for the cathode, allowing oxygen incorporation over the whole surface of the cathode, and ensuring the reaction is not restricted to the three-phase boundary. 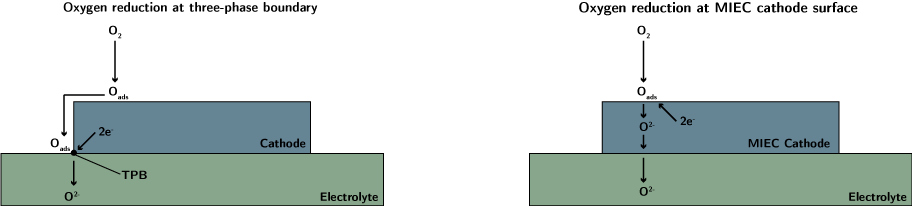
Perovskite cathode materialsPerovskite oxides are the main materials currently used as SOFC cathodes. The ideal perovskite structure, ABX3 is cubic, with a 12-coordinate A ion at the centre of the cell, and corner sharing BX6 octahedra. Sr-doped LaMnO3 is the conventional cathode material for high temperature operation due to its high electrical conductivity, good activity for the oxygen reduction reaction and compatibility with electrolyte materials. LaMnO3-based materials are under investigation for use at intermediate temperature. At intermediate temperature, LaMnO3 is distorted relative to the ideal cubic perovskite, due to Jahn-Teller distortion of the Mn-O bonds, giving two inequivalent oxygen sites, and GdFeO3-type distortion, causing rotation of the MnO6 octahedra. The formation energies of defects in orthorhombic LaMnO3 have been calculated using PBEsol + U, which has been found to give a good description of its structural and electronic properties. Oxygen diffusion in LaMnO3 occurs via oxygen vacancies. The formation of oxygen vacancies at chemical potentials relevant to SOFC operating conditions, in bulk LaMnO3 and at all low index surfaces have been examined. The oxygen vacancy formation energies at the surfaces of pure LaMnO3 are high, and found to vary significantly, Alkaline-earth doping may improve the mixed ionic and electronic conductivity of LaMnO3. The energies of formation of an alkaline earth defect has been investigated at both La and Mn sites, to establish the most probable site at which they will be introduced, under intermediate temperature SOFC operating conditions. The charge compensation mechanism for the introduction of alkaline earth dopants is under investigation, considering both ionic (formation of an oxygen vacancy for every 2 alkaline earth dopants introduced at a lattice site) and electronic compensation (a hole localised at the Mn site for each dopant introduced). 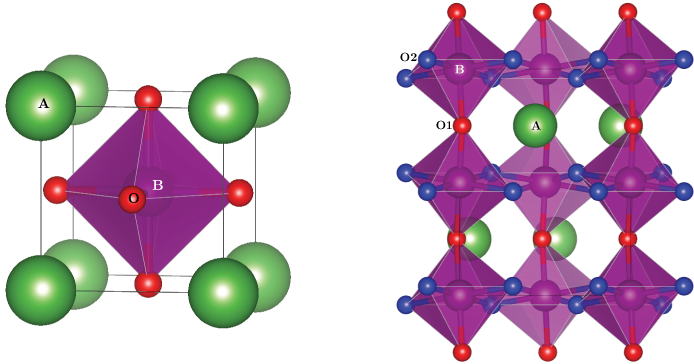
Ruddlesden-Popper cathode materialsRuddlesden-Popper (R-P) phases, An+1MnO3n+1, where A is a rare-earth/alkaline earth and M is a transition metal, which consist of n perovskite layers separated by rocksalt layers, have shown promise as cathode materials. These materials can accommodate an oxygen excess, so oxygen diffusion can occur via interstitial sites in the rocksalt layers. The main focus has been on Ruddlesden-Popper materials with n = 1, which possess the K2NiF4 structure. La2NiO4+δ has been suggested as a potential cathode material for intermediate temperature operation. Defect formation energies for oxygen vacancies and interstitials in La2NiO4+δ have been calculated using PBEsol + U. Oxygen vacancies were found to have significantly higher formation energies than oxygen interstitials, which have low formation energies under SOFC operating conditions. 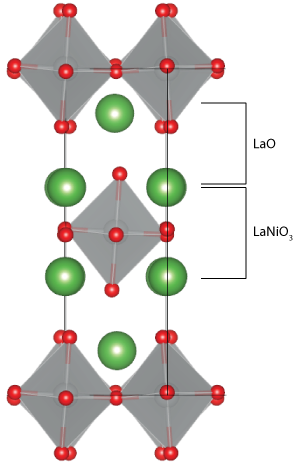
Email: watsong AT tcd.ie Last updated: May 25 2016 Back to Top |